MoCRA THE NEW US COSMETIC REGULATION
On December 29th, 2022, the Modernization of Cosmetics Regulation Act (MoCRA) was approved, which is the first major amendment to Chapter VI of the Federal Food, Drug and Cosmetic Act (FDCA) dating back to 1938.
It will come into effect on December 29th, 2023 and provides for the introduction of new obligations that companies selling cosmetic products in the United States will have to comply with.

US RESPONSIBLE PERSON/US AGENT: A KEY ROLE!
The most significant change concerns the requirement for an identified US Responsible Person, who must be listed on the label and be in contact with the FDA. Non-US resident companies can still fulfill this role, but they must appoint a local reference who possesses extensive expertise in interacting with the FDA, managing Adverse Events, and safeguarding confidential information. In this regard, we offer the role of US Agent, ensuring competence, professionalism, and confidentiality.
In addition to fulfilling legal obligations, we provide constant assistance and real-time updates, safeguarding the prosperity of your business in the USA!
The involved manufacturers themselves must proceed with the appointment of a US Agent who will handle the registration of facilities and obtain the FDA Establishment Identifier (FEI) number. Leveraging our expertise, we offer the role of US Agent to represent production sites, ensuring protection, competence, and discretion.
BULLET POINTS:
- Regulatory Expertise: leverage our in-depth knowledge of MoCRA and other relevant regulations to ensure compliance with US cosmetic requirements.
- FDA Liaison: serve as a direct point of contact with the FDA, facilitating communication and addressing any regulatory inquiries or concerns.
- Compliance Management: implement robust systems and processes to monitor and maintain compliance with all applicable regulations.
- Product Registration (product listing): assist with the registration process for cosmetic products, ensuring all necessary documentation is submitted accurately and on time.
- FEI number: as the appointed US Agent, we will provide the facilities with their FDA Establishment Identifier (FEI) number.
- Adverse Event Reporting: help navigate the adverse event reporting requirements outlined in MoCRA, ensuring timely and accurate reporting when necessary.

THE FUTURE OF COSMETICS IN THE USA
HOW TO EFFECTIVELY PREPARE FOR THE ARRIVAL OF MOCRA
• Methods and timelines for complying with new requirements
• Immediate actions and planning for the next steps
• Key changes: facility registration, product listing, Good Manufacturing Practices (GMP), safety assessment, new labeling requirements, reporting adverse events, recordkeeping
REGULATORY REFERENCES

Approved by the United States Congress on December 23, 2022
The MoCRA amendments to Chapter VI of the Federal Food, Drug, and Cosmetic Act (FDCA) represent the first major update to cosmetics regulations since 1938 and introduce 10 new sections in addition to the existing 3

The new law now grants the FDA increased authority over cosmetic companies
MOCRA: WHY?
1938
REGULATION
FRAGMENTED REGULATION
PRODUCT SAFETY NOT DEMONSTRATED AND NOT DOCUMENTED
POOR CONSUMER PROTECTION
FDA: LIMITED POWER OF ACTION


Safety Substantiation
Scientific demonstration of product and ingredient safety

Always supported and accompanied by the necessary documentation
Always valid and up-to-date, with constant and continuous updates
Through thorough studies, tests, analyses, trials, and information
↓
Toxicological data

Qualified Expert with documented competence and experience
↓
Safety Assessor
Safety Assessment
related to the product
and ingredients
↓
Toxicological data
U.S. Responsible Person
• Based in the U.S.A. or a US Agent can be appointed
• Single point of contact for authorities
• Registrations: product listing, facility registration
• Management of Serious Adverse Events
• Safety Substantiation
• Specific regulatory expertise
U.S. AGENT appointed by the manufacturer for registration of their facilities.
Carefully consider the appointment of a local distributor for confidentiality of product/supplier information, reliability of their regulatory and scientific expertise, experience and ability to communicate with authorities, and data storage capabilities.

REGISTRATIONS: NOT JUST FOR BRANDS
FACILITY REGISTRATION
a US Agent must be appointed
manufacturers and actors in the production chain
to be renewed every 2 years
updates within 60 days
Existing
register by December 29, 2023
New
register within 60 days of activation
REQUIRED INFORMATION
REFERENCES
RP CONTACTS
CATEGORIES
REGISTRATION NUMBER
BRAND
REGISTRATIONS: PRODUCTS AND INGREDIENTS
PRODUCT LISTING
U.S. Responsible Person
information on products and ingredients
to be updated annually
REQUIRED INFORMATION
REGISTRATION NUMBER
RP REFERENCES
CATEGORY
INGREDIENTS
PLD
LABELING
SAME REFERENCE FRAME AS BEFORE
Principal Display Panel (PDP) e Information Panel (IP)
PRODUCTS FOR PROFESSIONAL USE
Warning on the label
U.S. RESPONSIBLE
PERSON REFERENCES
Address, phone, email, website
FRAGRANCE ALLERGENS
delle fragranze
List to be defined
Serious Adverse Events
AE Management
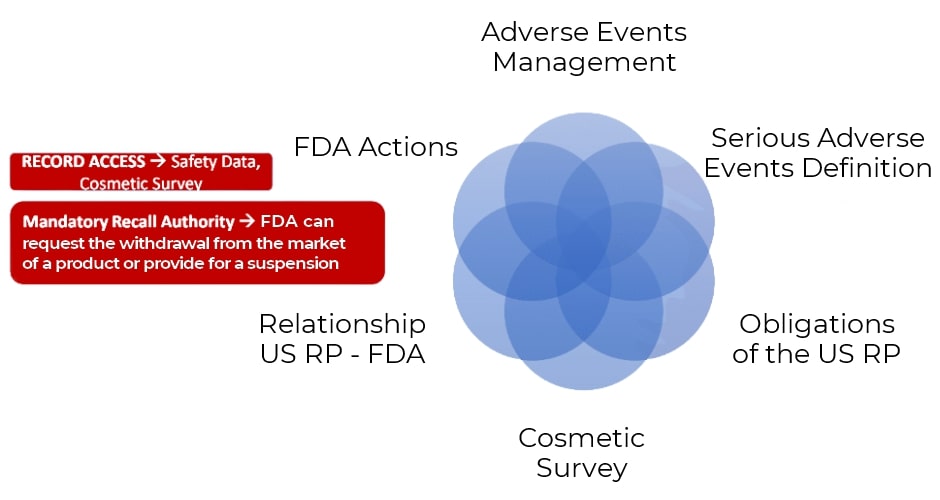
The U.S. Responsible Person must:
• submit a report and a copy of the product labeling within 15 days of receiving the notification
• medical update (New Medical Information): for 1 year after the event, within 15 days of receiving additional data
• receipt of notifications: at the physical address, email, and website of the US RP
• maintain records for 6 years
• provide access to authorized personnel from the Department of Health and Human Services
FDA: suspicion on fragrance components
• may request a list of all products containing them
• the U.S. Responsible Person must provide the list within 30 days
• può richiedere l’elenco di tutti i prodotti che le contengono
• la U.S. Responsible Person deve fornire l’elenco entro 30 giorni
Good Manufacturing
EXPECTED SUBSTANTIAL ALIGNMENT WITH ISO 22716
FINAL RULE BY DECEMBER 29, 2024
IN DEFINITION: DATA COLLECTION PHASE
OBLIGATION TO APPLY: DECEMBER 29, 2025
ACTION PLAN
WHAT TO DO NOW:
• identify a US Responsible Person/ US Agent
• organize the management of Adverse Effects (Cosmetic Survey)
• Safety Substantiation – documented, in English, up-to-date, data
• labeling – compliant
WHAT TO DO NEXT:
• product registration (with ingredient listing), manufacturers, and production chain actors
• inspections (GMP) by the FDA —> occhio che nella versione ITA l’FDA è scritto solo FD